Manufacturer Role
Manufacturer Role in Pricing Component of 340B OPAIS
The ![]() 340B OPAIS The 340B Office of Pharmacy Affairs Information System (OPAIS) is a collection of information submitted by covered entities, contract pharmacies, and manufacturers maintained and verified by HRSA's Office of Pharmacy Affairs (OPA). requires a separate login for access to pricing data even if you are already logged in as an AO or
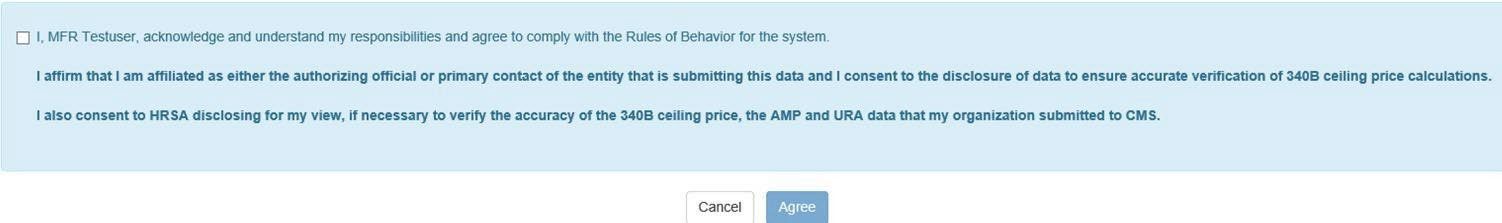
340B OPAIS The 340B Office of Pharmacy Affairs Information System (OPAIS) is a collection of information submitted by covered entities, contract pharmacies, and manufacturers maintained and verified by HRSA's Office of Pharmacy Affairs (OPA). requires a separate login for access to pricing data even if you are already logged in as an AO or ![]() PC External user who is designated as a Primary Contact for an entity. This user can enter registrations and update entity information. This user can enter registrations, and update entity information. Any changes to an entity performed by the PC user must be attested to by the AO for that entity. in the registration system. The system will prompt you to log in, authenticate, and agree to the Rules of Behavior again. The following attestation will be appended to the Rules of Behavior page. You must select the checkbox and click the Agree button to proceed.
PC External user who is designated as a Primary Contact for an entity. This user can enter registrations and update entity information. This user can enter registrations, and update entity information. Any changes to an entity performed by the PC user must be attested to by the AO for that entity. in the registration system. The system will prompt you to log in, authenticate, and agree to the Rules of Behavior again. The following attestation will be appended to the Rules of Behavior page. You must select the checkbox and click the Agree button to proceed.
Manufacturer Dashboard
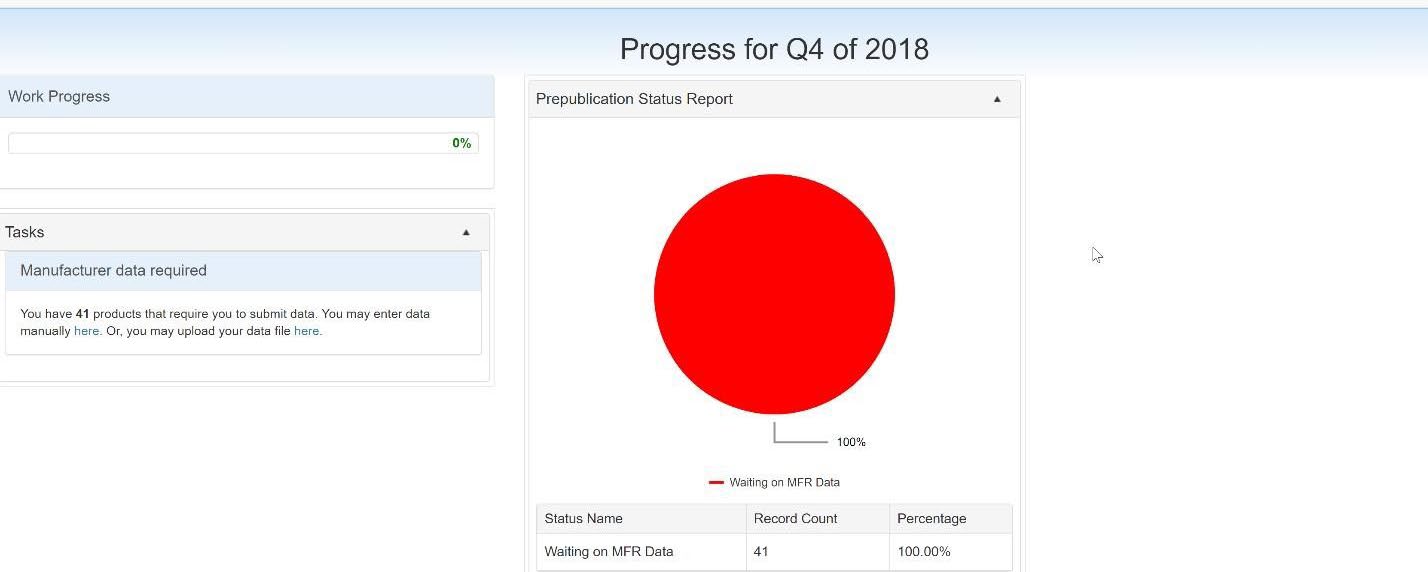
After a successful login, the Manufacturer 340B OPAIS Dashboard is displayed. The dashboard displays the progress made on the tasks either to provide or reconcile pricing data. Shortcut links are available to the tasks from the Tasks section and the prepublication status report.
Click the small arrow in the upper-right corner of the Tasks or Prepublication Status Report section to minimize their respective windows.
Price Corrections
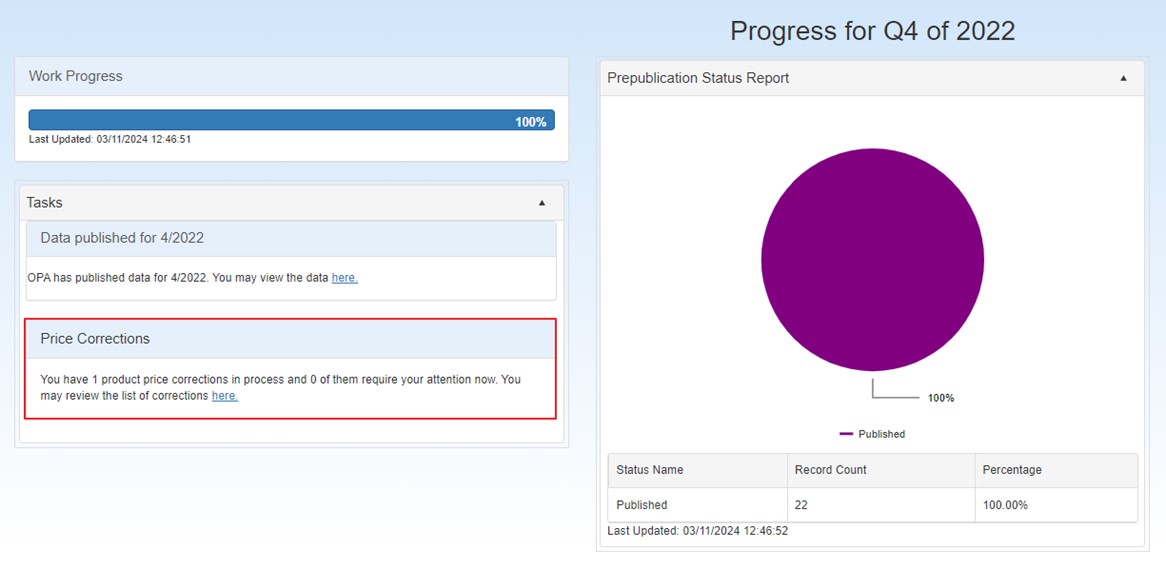
A new section for Price Corrections has been added to the Dashboard, which will be displayed if you have one or more price correction requests in process.
The Price Corrections section displays the following: "You have # product price corrections in process and # of them require your attention now. You may review the list of corrections here" and contains a link to the list of price corrections.
Menu Bar Navigation
The top menu bar displays the following menu options:
| Option | Description |
|---|---|
| Home | Displays the Manufacturer Dashboard page. |
| Upload | Upload a Manufacturer Instrument File. |
| Edit | Manually enter product data, review the submission status and reconcile tasks after the manufacturer instrument file upload. |
| Submit | Select this option to submit pricing data to OPA. This option is available after manufacturers have completed all the data submission and reconciliation tasks. The work progress bar will show 100% when all tasks are complete. |
| Search | Displays the Product Search page to locate products and view historical published quarterly pricing data details. You can create and submit price correction requests from the Product Detail page. |
| Reports | Select the Reports link from the top menu to view the pricing information from the current pricing quarter that is under OPA adjudication as well as from the previously published quarters for the NDCs associated wiht your labeler codes for the previous 12 quarters. There is an option to Export the pricing information to Excel available on this page. |
| Corrections | Select this option to view the in-progress Price Corrections that are under review. |
| Logged in user email address | Click on the email address and select the Logout option from the drop- down menu to logout of the system. |